上海金畔生物科技有限公司代理AAT Bioquest荧光染料全线产品,欢迎访问AAT Bioquest荧光染料官网了解更多信息。
Cell Meter Beta-Arrestin蛋白质易位GPCR信号检测试剂盒
 |
货号 | 36390 | 存储条件 | 在零下15度以下保存, 避免光照 |
| 规格 | 100 Tests | 价格 | 9180 | |
| Ex (nm) | Em (nm) | |||
| 分子量 | 溶剂 | |||
| 产品详细介绍 | ||||
简要概述
所有G蛋白偶联受体(GPCR)在通过配体结合激活后,都会通过一条共同途径迅速发生脱敏作用。 β-arrestin(一种细胞质蛋白)与激活的受体的结合使GPCR信号失活,并启动了受体向细胞内的易位,在此细胞中配体被去除,受体被循环回到细胞膜。通过将荧光标记(例如GFP)附着到β-arrestin,可以检测受体arrestin复合物的位置。由于脱敏仅发生在激活的受体上,因此检测β-arrestin的易位和随后的受体再循环提供了检测GPCR靶标激活的可靠方法。 Cell Meter Beta-Arrestin蛋白易位GPCR信号试剂盒提供了功能强大的功能分析,可通过荧光成像筛选目标化合物针对已知或孤立GPCR靶标的活性。靶向GPCR的激活诱导荧光向细胞膜和内吞囊泡的移位。
适用仪器
| 荧光显微镜 | |
| 激发: | FITC滤波片 |
| 发射: | FITC滤波片 |
| 推荐孔板: | 黑色透明底板 |
产品说明书
样品实验方案
概述
准备细胞进行转染
准备Transfectamine 5000-DNA混合物
将Transfectamine 5000-DNA混合物添加到细胞培养物中,并孵育过夜
转染后24-30小时将转染的细胞转移到96孔板中,并将培养物孵育过夜
在荧光显微镜下分析由GPCR激活引起的转运
细胞准备
细胞密度在转染时它们将达到约60-70%的融合度。
转染前用新鲜的生长培养基替换。
注意:例如,对于6孔板,每孔替换为2 mL培养基,对于10 cm板,则替换为6 mL培养基。
储备溶液配制
除非另有说明,否则所有未使用的储备溶液应分为一次性使用的等分试样,并在制备后储存在-20°C下。避免重复冻融循环。
β-arrestin-GFPDNA储备溶液
向小瓶β-arrestin-GFPDNA(组分A)中加入10 µL ddH2O,充分混合至终浓度为1 µg / µL。
操作步骤
1.将3 µg DNA(例如1.5 µg Beta-arrestin-GFP DNA储备液和1.5 µg您准备的GPCR DNA)与200 µL无血清培养基混合。
2.向混合物中加入9 µL Transfectamine 5000(组分B)。
3.充分混合并在室温下孵育20分钟。
注意:Transfectamine 5000和DNA的比例需要针对不同的细胞系进行优化,通常,在我们的测试中,Transfectamine 5000转染试剂(µL)与DNA(µg)的比例应为3-5 µL:1ug。
表1. 6孔板和10 cm板的样本表
| 6孔板(每孔) | 10cm板 | |
| 新鲜培养基 | 2 mL | 6 mL |
| 质粒 | 3 µg | 10 µg |
| 无血清培养基 | 200 µL | 600 µL |
| Transfectamine 5000转染试剂 | ~9 µL | ~30 µL |
转染和转运方案
1.将Transfectamine 5000 -DNA混合物添加到培养板中,并孵育过夜。
注意事项:最早在转染后16小时即可检测到重组蛋白。转染后72〜96小时可观察到最大表达水平。
2.转染后24-30小时将转染的细胞转移到96孔板中,并孵育过夜。
使用FITC滤光片(Ex / Em = 488/530 nm)在荧光显微镜下检测受体激活诱导的β-arrestin易位。
图示
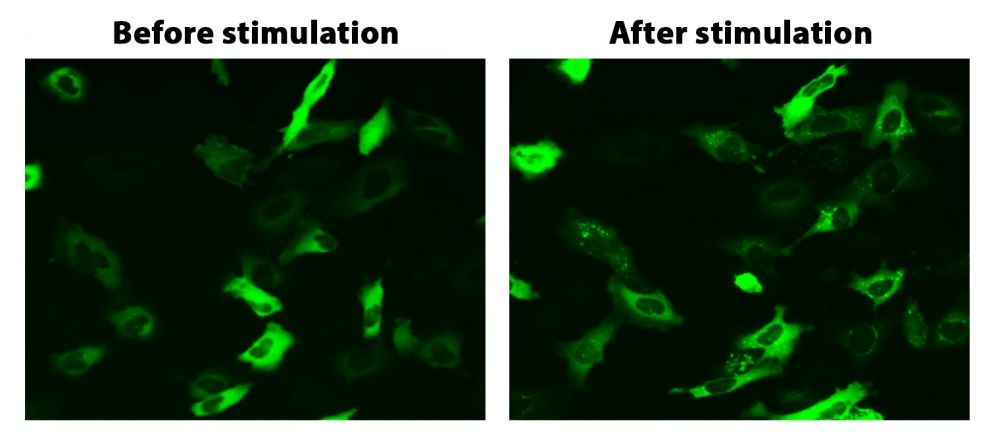
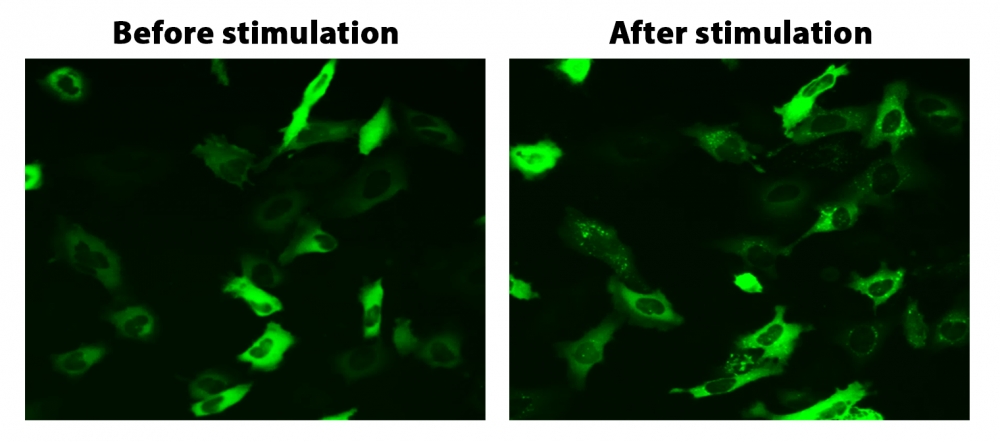
图1. HeLa细胞中β-arrestin的易位。 HeLa细胞用β-arrestin-GFP和加压素受体2(V2R)瞬时转染。 HeLa细胞在6孔板中培养,并融合至〜60%。用9 µL Transfectamine 5000转染等量的β-arrestin-GFP(1.5 µg)和V2R质粒(1.5 µg)。转染后约30小时,将细胞转移到96孔板中。转染后约48小时,将加压素(1 µM)加入细胞中以诱导β-arrestin-GFP易位。使用FITC通道在荧光显微镜下加压素处理之前和之后2小时拍摄图像。
参考文献
Screening cellular feature measurements for image-based assay development.
Authors: Logan, David J and Carpenter, Anne E
Journal: Journal of biomolecular screening (2010): 840-6
Kappa opioid receptor screen with the Tango beta-arrestin recruitment technology and characterization of hits with second-messenger assays.
Authors: Doucette, Christopher and Vedvik, Kevin and Koepnick, Elizabeth and Bergsma, Aaron and Thomson, Brian and Turek-Etienne, Tammy C
Journal: Journal of biomolecular screening (2009): 381-94
Multiplexed assays by high-content imaging for assessment of GPCR activity.
Authors: Ross, D A and Lee, S and Reiser, V and Xue, J and Alves, K and Vaidya, S and Kreamer, A and Mull, R and Hudak, E and Hare, T and Detmers, P A and Lingham, R and Ferrer, M and Strulovici, B and Santini, F
Journal: Journal of biomolecular screening (2008): 449-55
Comparison of G-protein coupled receptor desensitization-related beta-arrestin redistribution using confocal and non-confocal imaging.
Authors: Haasen, Dorothea and Wolff, Michael and Valler, Martin J and Heilker, Ralf
Journal: Combinatorial chemistry & high throughput screening (2006): 37-47
High-content screening of known G protein-coupled receptors by arrestin translocation.
Authors: Hudson, Christine C and Oakley, Robert H and Sjaastad, Michael D and Loomis, Carson R
Journal: Methods in enzymology (2006): 63-78
High-throughput confocal microscopy for beta-arrestin-green fluorescent protein translocation G protein-coupled receptor assays using the Evotec Opera.
Authors: Garippa, Ralph J and Hoffman, Ann F and Gradl, Gabriele and Kirsch, Achim
Journal: Methods in enzymology (2006): 99-120
The ligand-independent translocation assay: an enabling technology for screening orphan G protein-coupled receptors by arrestin recruitment.
Authors: Oakley, Robert H and Hudson, Christine C and Sjaastad, Michael D and Loomis, Carson R
Journal: Methods in enzymology (2006): 50-63
Development and validation of algorithms for measuring G-protein coupled receptor activation in cells using the LSC-based imaging cytometer platform.
Authors: Ozawa, Kazuo and Hudson, Christine C and Wille, Kirsten R and Karaki, Sachiko and Oakley, Robert H
Journal: Cytometry. Part A : the journal of the International Society for Analytical Cytology (2005): 69-76
Quantitative cell-based high-content screening for vasopressin receptor agonists using transfluor technology.
Authors: Ghosh, Richik N and DeBiasio, Richard and Hudson, Christine C and Ramer, Everett R and Cowan, Conrad L and Oakley, Robert H
Journal: Journal of biomolecular screening (2005): 476-84
The cellular distribution of fluorescently labeled arrestins provides a robust, sensitive, and universal assay for screening G protein-coupled receptors.
Authors: Oakley, Robert H and Hudson, Christine C and Cruickshank, Rachael D and Meyers, Diane M and Payne, Richard E and Rhem, Shay M and Loomis, Carson R
Journal: Assay and drug development technologies (2002): 21-30
